-
tel:
+86-18961602506 -
email:
fly03@flynewenergy.com
Dry information | The mystery of low-temperature performance of lithium-ion batteries: the game between particle size, electrolyte and diffusion speed
Dec 28, 2023
Below 0°C, the energy density and power density of lithium-ion batteries decrease rapidly. At low temperatures below -20°C, the performance of the battery deteriorates significantly. At -40°C, the battery can only discharge 30% or even less of the rated capacity. . Especially for lithium iron phosphate batteries, the material itself is an insulator, with low electronic conductivity, poor lithium ion diffusivity, and poor conductivity at low temperatures, which increases the internal resistance of the battery and is greatly affected by polarization. The charging and discharging of the battery are hindered, so low temperatures Performance is not ideal.
In order to further demonstrate the factors affecting the low temperature of lithium-ion batteries, this paper discusses the impact of lithium iron phosphate materials with different primary particle sizes on the low-temperature performance of lithium batteries, the impact of artificial graphite anodes with different particle sizes on the low-temperature performance of lithium batteries, and the impact of different primary particle sizes on the low-temperature performance of lithium batteries. Effects of the type and content of carboxylic acid ester solvent electrolytes on the low-temperature performance of lithium batteries.
1 experiment
1.1 Battery preparation
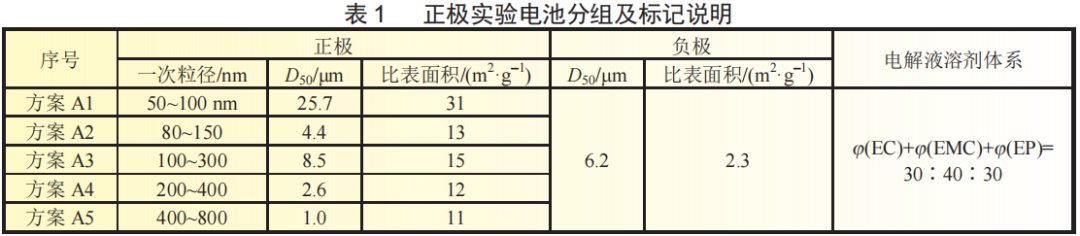
The positive active material used in the experiment is lithium iron phosphate with different primary particle sizes. The negative active material is carbon-coated artificial graphite with different D50. The electrolyte is 1.1 mol/L LiPF6/different solvent ratios and types. The selected solvents are: EC, EMC, DMC, EP, MP, PP, PA, the additive is 2%VC+1%PC+1%FEC. The specific parameters and program implementation are detailed below.
Mix five kinds of lithium iron phosphate materials with different primary particle sizes, the conductive agent Super P, the binder PVDF, and the dispersant PVP according to the mass ratio of 93.8:3.5:2.5:0.2, and use NMP as the solvent to form a positive electrode slurry. Coated on 15μm carbon-coated aluminum foil, design different coating amounts according to the specific capacity of the active material to ensure the same capacity per unit area, rolling, tableting and baking (110℃@24h).
Carbon-coated artificial graphite with different D50 is mixed with the conductive agent Super P, CMC and SBR according to the mass ratio of 94.4:2.0:1.6:2.0, and deionized water is used as the solvent to form a negative electrode slurry, which is coated on a 6 μm copper foil On the surface, different coating amounts are designed according to the specific capacity of the active material withholding mass, so as to ensure the same capacity per unit area, rolling, tableting, and baking (100℃@24h).
Assemble the positive and negative electrode sheets and separator stacks into a battery core, dry it and then inject electrolyte. The electrolyte formula is 1.1 mol/L LiPF6, the solvent system and type are different, and the additives are 2%VC+1%PC+1%FEC. After leaving it aside, the battery is first formed with a current of C/10, and then charged and discharged with a current of C/5 and C/2 to form a stable SEI film. See below for battery preparation protocol.
1.2 Analysis of physical and chemical properties of materials
Use a laser particle size analyzer to test the particle size of the material; use a surface area meter to test the surface area of the material. Scanning electron microscopy was used to characterize the morphology and primary particle size of the lithium iron phosphate cathode.
1.3 Electrical performance test
Low-temperature discharge test: In an environment of normal temperature (25±2)℃, the experimental battery is charged at a constant current of 1C to 3.65V, then charged at a constant voltage to 0.05C, left for 10 minutes, then discharged at a rate of 1C to 2.0V, and cycled according to the above steps 3 times, ending with full charge. After being left in a low temperature (-40±2)°C environment for 18 hours, discharge it to 2.0V at 0.2C, and record the discharge capacity and median discharge voltage.
Low-temperature charging test: First, under normal temperature (25 ± 2) ℃, the experimental battery is charged to 3.65V at a constant current of 1C, then charged to a constant voltage of 0.05C, left for 10 minutes, and then discharged to 2.0V at a rate of 1C. Follow the above The steps are repeated three times and end in the discharge state. Secondly, after leaving it in a low temperature (-20±2)℃ environment for 18 hours, charge it to 3.65V at a constant current of 0.5C, then charge it to a constant voltage of 0.05C, leave it for 10 minutes, and then discharge it to 2.0V at a rate of 0.5C. Follow the above steps. Cycle 3 times and record the low temperature charging capacity.
2 Results and discussion
2.1 The impact of primary particle size of lithium iron phosphate on low-temperature performance of batteries
As can be seen from the SEM electron microscope image in Figure 1, the primary particle sizes of the five types of lithium iron phosphate are 50~100nm, 80~150nm, 100~300nm, 200~400nm, and 400~800nm.
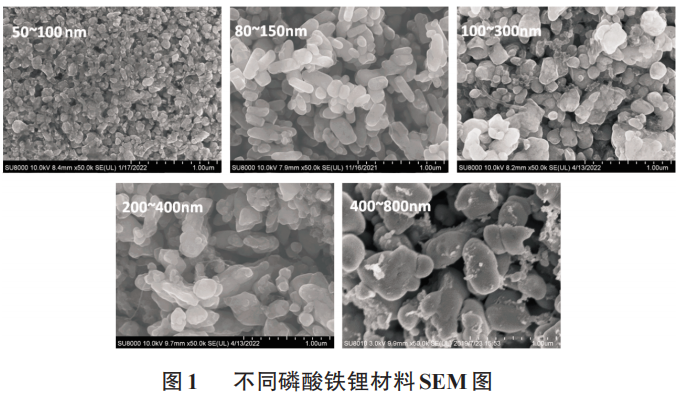
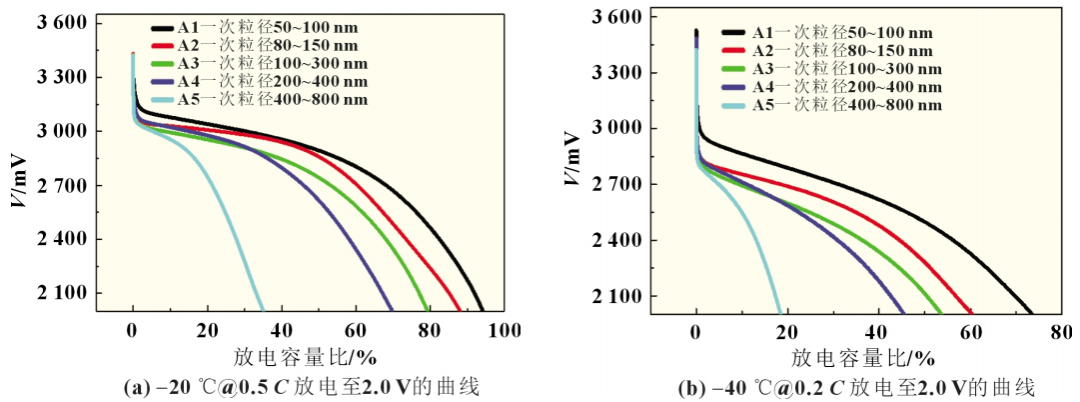
Obviously, as the primary particle size of the positive electrode lithium iron phosphate increases, the lithium ion diffusion path becomes longer, which is not conducive to the performance of low-temperature performance. That is, when lithium ions diffuse on the surface and inside the larger primary particle size, a higher concentration of electrodes is generated. polarization, while the concentration polarization produced on the surface of smaller primary particles and during internal diffusion is lower. Higher concentration polarization reduces the battery voltage, reaching the lower limit cut-off voltage well before each particle reaches its available capacity, shortening the discharge time, and reducing the available capacity at low temperatures. Experimental data show that the capacity decreases proportionally at low temperatures, which shows that the primary particle size of the cathode active material plays a key role in the low-temperature discharge performance of the battery.
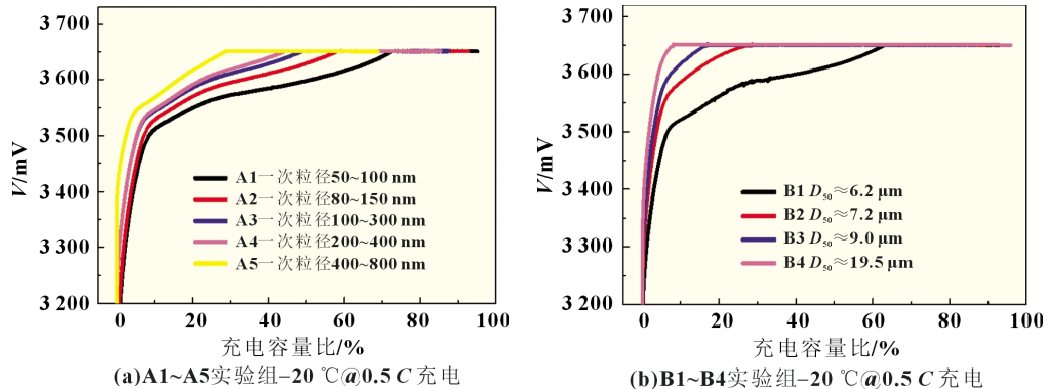
Low-temperature charging test: After the experimental battery was left in an environment of (-20±2) ℃ for 18 hours, it was charged to 3.65V at 0.5C. The charging curve is shown in Figure 3(a). The constant current ratio of experimental batteries in Schemes A1 to A5 dropped from 0.76 to 0.41, and the constant current charging capacity ratio dropped from 72.0% to 28.2%. It can be seen that even during low-temperature charging, the primary particle size of the positive active material still has a greater impact on the low-temperature charging performance of the battery. This may be due to the fact that the lithium iron phosphate with smaller particle size is delithiated more uniformly at low temperature, resulting in smaller concentration polarization, and the positive electrode potential rises more slowly, that is, a longer constant current charging time is required to reach 3.65 The cut-off voltage is V, so the lithium iron phosphate battery with a smaller primary particle size can charge more power when it reaches the cut-off voltage. It may also be due to the fact that during charging, the lithium iron phosphate with a smaller primary particle size is delithiated relatively uniformly, and the polarization generated after reaching the negative electrode graphite layer is small. The negative electrode potential decreases slowly, and the lithium-ion battery voltage rises slowly, and the constant current Longer time and high charging capacity.
Experiments in Scheme A show that during low-temperature discharge, the smaller the primary particle size of the cathode active material, the shorter the corresponding lithium ion diffusion path, the larger the diffusion coefficient, the lower the battery voltage decrease, and the longer each particle needs to reach its available capacity. It takes a long time to reach the lower limit cut-off voltage, the discharge time is long at low temperature, and the available capacity is higher. During low-temperature charging, lithium iron phosphate with a smaller primary particle size is more uniformly delithiated, and the voltage rises slowly, that is, a longer constant current charging time is required to reach the cut-off voltage. Therefore, lithium iron phosphate with a smaller primary particle size When the battery reaches the cut-off voltage, more electricity is charged, and during the charge and discharge process, the available capacity of experimental batteries with different primary particle sizes of lithium iron phosphate varies greatly. Obviously, the deintercalation speed of lithium ions on the surface and inside of the cathode active material plays a key role in the low-temperature performance of the battery.
2.2 Effect of graphite particle size on battery low temperature performance
A laser particle size analyzer and a specific surface meter were used to test the particle size and specific surface area of the negative active material. The D50 and specific surface area parameters are shown in Table 2. According to the order of D50 from small to large, B1~B4 experimental batteries were prepared with lithium iron phosphate with a primary particle size of 80~150nm and EP system electrolyte.
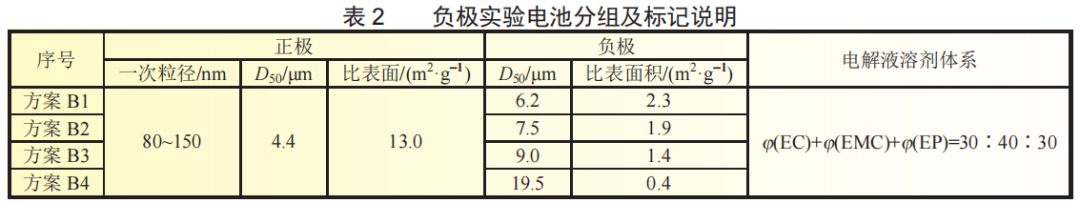
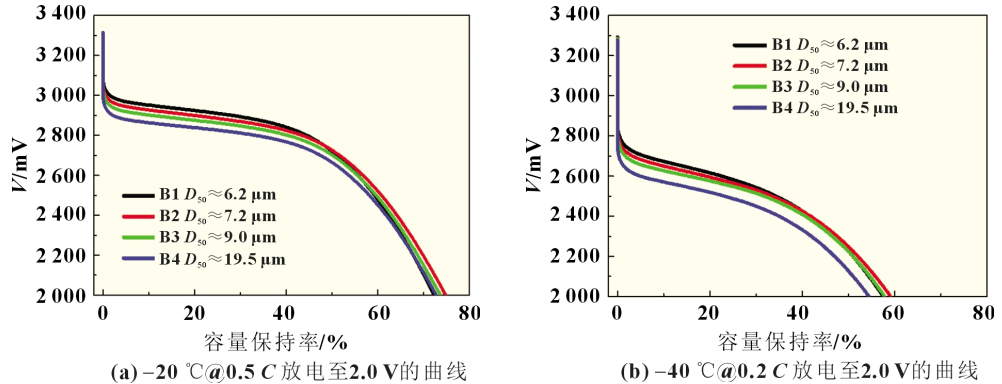
Low-temperature charging test: After the experimental battery was left in an environment of (-20±2)°C for 18 hours, it was charged to 3.65V at 0.5C. The charging curve is shown in Figure 3(b). The constant current ratio of experimental batteries B1~B4 dropped from 0.67 to 0.09, and the constant current charging capacity ratio dropped from 63.0% to 8.2%. From the data point of view, the constant current ratio or constant current charging capacity has dropped significantly. This may be due to the fact that at low temperatures, the graphite negative electrode with smaller particle size is more uniformly embedded with lithium, the polarization generated on the surface and inside the negative electrode particles is smaller, the excess electrons in the positive electrode are relatively small, the positive and negative electrode potential changes slowly, and the lithium-ion battery voltage The rate of increase is slower, that is, it takes longer constant current charging time to reach the cut-off voltage of 3.65V, and more power is charged. However, compared with the discharge capacity at -20℃@0.5C, the constant current charging capacity of the battery in experimental group AB at -20℃@0.5C decreased significantly. This is because lithium ions are detached from LiC6 when discharging a lithium-ion battery. This process is an exothermic reaction. However, when charging, lithium ions and carbon need to absorb heat to form LiC6, which may cause the electrochemical balance to shift to the lithiated state, making the charging process longer. Disaster. Therefore, the charge transfer resistance is greatly affected by the electrode state of charge (SOC), that is, the charge transfer resistance of a discharged lithium-ion battery is greater than that of a fully charged lithium-ion battery when it is discharged.
Experiments in Scheme B show that during low-temperature discharge, the smaller the particle size of the negative active material, the shorter the corresponding lithium ion diffusion path, the larger the diffusion coefficient, the lower the battery voltage decrease, and the longer it takes each particle to reach its available capacity. The time to reach the lower limit cut-off voltage, the discharge time at low temperature is long, and the available capacity is high. During low-temperature charging, the graphite negative electrode with smaller particle size is more uniformly embedded with lithium, the negative electrode particles and the polarization generated inside are smaller, the excess electrons in the positive electrode are relatively small, the positive and negative electrode potential changes slowly, and the lithium-ion battery voltage rises at a very fast rate. Slower, that is, it takes longer constant current charging time to reach the cut-off voltage of 3.65V, and more power is charged. However, compared with the discharge process, the size of graphite particles has a smaller impact on the low-temperature discharge capacity of lithium-ion batteries. Therefore, it can be said that the insertion speed of lithium ions on the surface of the negative electrode graphite particles plays an important role in the low-temperature charging performance of the battery.
2.3 Effect of electrolyte on battery low-temperature performance
The electrolytes of different solvent systems listed in Table 3 were sequentially injected into the cells of lithium iron phosphate with a primary particle size of 80~150nm and carbon-coated artificial graphite with a D50 of 6.2μm. The experimental group batteries were labeled C1~C7.
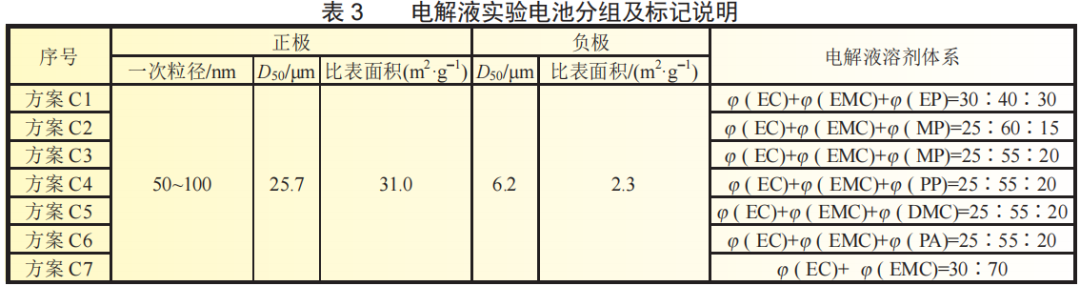
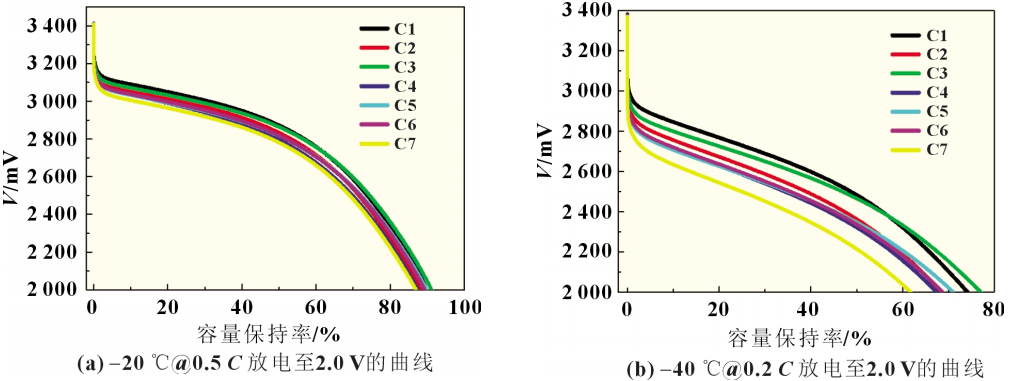
As can be seen from Figure 5, the low-temperature performance of the experimental batteries of Schemes C1, C2, and C3 is better. This is due to the lower viscosity and higher conductivity of the co-solvents containing MP and EP. The faster the ion conduction rate, the greater the polarization. The smaller it is, the better the battery performs at low temperatures. In general, the conductivity of co-solvents containing small molecule esters will drop 4 to 5 times at -40°C, but is still about 3 times higher than the conductivity of electrolyte 5.
The experiment in Scheme C shows that introducing a low-viscosity co-solvent into the electrolyte can increase the conductivity of the electrolyte, speed up the ion conduction rate, reduce concentration polarization, and help improve the discharge capacity of lithium-ion batteries at low temperatures. However, compared with the multiple increase in conductivity by the co-solvent, the capacity improvement is smaller. Obviously, the conductivity of the electrolyte is not the main factor affecting the low-temperature performance of the lithium-ion battery at this time.
3 Conclusion
By studying the impact of different positive electrodes, negative electrodes and electrode solutions on low-temperature performance of experimental batteries, it was found that the use of graphite with smaller particle sizes and electrolytes containing carboxylic acid ester solvents is beneficial to improving the low-temperature discharge performance of the battery, but it is inconsistent with the positive electrode active material. Compared with the impact of primary particle size on low-temperature discharge, the impact of the two is relatively weak. Taking -40℃@0.2C low-temperature discharge as an example, the difference between the highest discharge capacity ratio and the lowest discharge capacity ratio in the scheme B group is 3%, the difference between the highest discharge capacity ratio and the lowest discharge capacity ratio in the scheme group C experiment is 15%, and the difference between the highest discharge capacity ratio and the lowest discharge capacity ratio in the scheme group A is 15%. The experimental discharge capacity ratio range is 72.0% to 28.2%, and the capacity ratio has the highest decrease ratio. That is, the primary particle size of the positive active material plays a leading role in the low-temperature discharge performance of the battery. At the same time, by comparing the low-temperature charging data of positive and negative electrode experimental batteries, it was found that the particle size of both positive and negative electrodes will have a greater impact on low-temperature charging performance. Obviously, reducing the primary particle size of the positive electrode or the graphite particle size of the negative electrode shortens the lithium ion diffusion path and reduces polarization, which is beneficial to the low-temperature performance of the battery. Therefore, it is further proved that the diffusion of lithium ions in the electrode surface layer and the electrode body is the main reason affecting the low-temperature performance of lithium-ion batteries. That is, the primary particle size of the positive electrode and the particle size of the negative electrode graphite play a key role in the low-temperature performance of the battery, especially the positive electrode. The primary particle size of the active material plays a dominant role in the low-temperature discharge performance of the battery.







